Chemistry Quiz Questions-(Chemical and elements)
Category - Basic Chemistry I
|
||
Question
|
Correct Answer
|
|
An atom is generally regarded as being made up of
|
Electrons Neutrons Protons
|
|
An element is made up of
|
Atoms
|
|
What forms the nucleus of the atom ?
|
Protons Neutrons
|
|
What particles move rapidly around the nucleus ?
|
Electrons
|
|
A pure substance that cannot be split up by a chemical reaction is a
|
Element
|
|
What particle is by far the lightest ?
|
Electron
|
|
Mass Number - Atomic Number =
|
Number of Neutrons
|
|
Mixtures of elements combine to form a
|
Compound
|
|
The mass number is the number of what in an atom ?
|
Protons Neutrons
|
|
The Atomic Number is the total number of what in an atom ?
|
Protons
|
|
GENERAL QUESTIONS :
•H2O is liquid, but H2S is what?
Gas
Prismatic
• Gold paint made from:
Copper
• Name a reducing agent:
Hydrogen sulfide
• Name the dehydrating agent:
Sulfuric acid
• Name a bleaching agent:
Sulfur di oxide
• A rain coat is made up of what?
Polychloroethene
• Which element on adding to natural rubber makes it less sticky in hot weather and less hard in cold weather?
Sulfur
• Which chemical causes Minimata disease?
Mercury
• The absence of cobalt in minute quantities in human body causes what?
Pernicious anemia
• Which element can easily form chains?
Carbon
• Oxygen can accept electron from all elements except what?
Fluorine
• Which element is used as an antichlor?
SO2
• Which is the most reactive element in sixth group?
Oxygen
• Which is the smallest atom in sixth group element?
Oxygen
• All the oxide which contains two atoms of oxygen in a molecule is called what?
Di oxides
• Write example for slow chemical reaction:
Rusting of iron
Change of mill into curd
• Which catalyst used in the manufacture of ammonia from nitrogen and hydrogen?
Iron
• Which substance used as catalyst in the preparation of oxygen from potassium chlorate?
Manganese dioxide
• Which compound formed when hydrogen peroxide decomposes?
Water
• Which useable substance formed as a result of collision?
Active complexes
• Which element can toxic to plants growing in soils that are high acidity?
Aluminum
• Glass is made out of what?
Sand
• Which is considered to be an anomalous compound?
Water
• How would you know that a chemical is pure or not?
By checking its melting point
• Which drug is present in cola drinks?
Caffeine
• Which fuel produce maximum heat per gram burnt?
Hydrogen
• Which element in radioactive form is used for determining the age of artifacts, relics, bones etc. of the past?
Carbon
• Which product of living organisms was the first to be made under the laboratory conditions?
Urea
• Which drug is present in tobacco?
Nicotine
• What is the most common natural source for sulfur?
Volcanic region
• The first scientific definition of a chemical element was made in which book?
The Sceptical Chymist
• Which element is present in the least amount in a living body?
Manganese
• Which substance is produced when nitrogen react with hydrogen?
Ammonia
• Which metal has the density is less than that of water?
Sodium
• Which catalyst used in the manufacture of sulfuric acid?
Vanadium pent oxide
• Which metal react with water and forms an alkaline compound?
Sodium
• Which metal floats on water?
Sodium (potassium)
• Which theory is used for explaining the changes in reaction rate?
Collision theory


 - that is the probability of molecule to have energy greater than or equal to E at the given temperature T. This exponential dependence of a reaction rate on temperature is known as the
- that is the probability of molecule to have energy greater than or equal to E at the given temperature T. This exponential dependence of a reaction rate on temperature is known as the 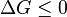 ; if it is equal to zero the chemical reaction is said to be at
; if it is equal to zero the chemical reaction is said to be at